Frequently Asked Questions
About IVI
The International Vaccine Institute (IVI) is a nonprofit international organization established in 1997 as an initiative of the United Nations Development Programme (UNDP).
Our mission is to discover, develop and deliver safe, effective and affordable vaccines for global public health.
IVI accelerates vaccine research and development to protect the world’s most vulnerable people from infectious diseases and enable healthy, productive lives.
With an innovative development model and public-private partnerships, IVI focuses on vaccines that are critically needed in resource-limited settings but often overlooked by major commercial developers.
Learn more about our approach here.
IVI boasts a diverse, multidisciplinary workforce of biomedical scientists, infectious disease experts, medical doctors and nurses, health economists, vaccine advocates, educators, communicators, and more representing 20 nationalities.
Meet our executive leadership team here.
Explore our career opportunities here.
IVI is based in Seoul, Republic of Korea with active field programs in 24 countries across Asia, Africa, and Latin America.
Explore the map of our programs here.
IVI works closely with governments, vaccine developers and manufacturers, philanthropies and foundations, international organizations and public-private partnerships, policymakers, universities, hospitals, and businesses to make safe, effective, and affordable vaccines available for global public health.
Four governments provide core funding for IVI: The Republic of Korea, Sweden, India, and Finland. Much of IVI’s work comes from research grants written by scientists at IVI and their collaborators. These grants come from the Bill & Melinda Gates Foundation, the Wellcome Trust, CEPI, the Samsung Life Public Welfare Foundation, and the Korea Support Committee for IVI, as well as academic institutions, industry partners, and individuals.
IVI does not directly fund grants for research and development to other organizations or individuals. However, IVI often awards subcontracts to external collaborators for select projects.
- Antigen discovery, pre-clinical development of new vaccines, development and standardization of laboratory assays in support of vaccine R&D with expertise in molecular, clinical, and translational immunology; vaccine process development; animal studies; and biosafety
- Public health, including epidemiology, interventions and implementation research, policy and health economics, biostatistics, and data management
- Clinical development and operations, and regulatory affairs
- Vaccination campaigns and vaccine effectiveness studies
- Quality management
- Project and coalition management
- Governmental affairs, communications, and advocacy
IVI is uniquely present at every step of the vaccine value chain. From public health research at field sites around the world to preclinical discovery and development in the lab, clinical trials and efficacy studies, technology transfers and registration with manufacturers, and vaccination campaigns in outbreak settings, IVI is there to make vaccines more available and accessible to the world’s most vulnerable people.
IVI is an international organization like the United Nations or the World Bank. Founded with an establishment agreement at the United Nations in 1997, IVI has 36 countries and the WHO as member states with 4 countries providing core funding. A representative from each funding member state sits as a voting member on the Board of Trustees.
More information on IVI’s member states may be found here.
IVI is prohibited from manufacturing vaccines. Instead, we discover new vaccines, improve existing ones, and transfer vaccine technology to manufacturers for vaccine production.
An oral cholera vaccine and typhoid conjugate vaccine were developed in-house at IVI laboratories, and IVI is currently investing in preclinical development of two vaccine candidates for non-typhoidal Salmonella and Shigella.
In addition to its own vaccines, IVI assists vaccine developers around the world with pre-clinical and clinical vaccine development.
With an initial focus on cholera, typhoid, and dengue, IVI has since expanded disease burden studies and vaccine R&D programs to include adenovirus type 55, chikungunya, COVID-19, group A strep, hantavirus, hepatitis viruses, HIV, human papillomavirus (HPV), MERS, salmonella, schistosomiasis, severe fever with thrombocytopenia syndrome virus, shigella, and tuberculosis (TB).
IVI also has three separate programs to increase the available volume of data for global antimicrobial resistance (AMR).
Learn more about IVI’s disease areas here.
About Vaccines
Vaccines train the body’s immune system to recognize and fight pathogens like viruses and bacteria.
When you receive a vaccine, you introduce your body to a piece of the germ or a version of a germ that won’t harm you but will trigger an immune (or defensive) response. So, if you come across the “real thing” in the future, your body will quickly remember how to protect against the potentially dangerous germ. This is called immunological memory.
Vaccines save lives. Immunization saves 2.5 million people every year and millions more from illness and disability according to the WHO. Vaccines have eliminated or eradicated dangerous infectious diseases from significant regions of the global map, protect against cancer, reduce the spread of antimicrobial resistance, and help mitigate the health ramifications of climate change.
Beyond health benefits, vaccination enables children to perform better in school and adults to continue working without the burden of preventable disease, reduces poverty, and increases equality and access to opportunities.
Learn more about the full value of vaccines here.
Vaccines have two major types of ingredients: antigens, which create an immune or “defensive” response in your body by producing protective antibodies, and adjuvants, which help boost that immune response. Other ingredients may be preservatives and residual antibiotics to prevent contamination, and stabilizers to keep vaccines potent after manufacturing.
Vaccine development begins in the lab with scientists creating a vaccine candidate. There are 6 main types of vaccines: live attenuated (which contain whole bacteria or viruses, though weakened), inactivated (with killed bacteria or viruses, or small parts of them), subunit (which contain only pieces of the bacteria or virus), conjugate (which connect pieces from the bacteria’s coating to antigens), toxoid (which only contain the part of the pathogen that causes disease), and viral vector-based vaccines (which use a harmless virus to deliver genetic code for antigen).
The new vaccine then enters pre-clinical research which includes testing in animals to see if the right kind of immune responses are generated. Then, testing in humans proceeds in three phases:
Phase I primarily tests for safety while also looking for the antibody responses. This earliest stage of testing in humans recruits a small number of healthy adults.
Phase II is larger and focuses on those amount of antibody responses. These trials often run for 1 year or longer with hundreds of volunteers, followed by safety analysis.
Phase III proves that a vaccine is safe and protects against disease. This final stage of clinical testing often involves thousands of people with a portion receiving a placebo.
Following clinical development, results are submitted for extensive review and ultimately approval by regulatory authority (e.g., MFDS in Korea), followed by WHO prequalification.
On a traditional timeline, it takes on average 5-10 years to take a vaccine from the laboratory to a vaccination program. Every stage from discovery through regulatory review can take at least two years, sometimes up to five. Once a vaccine proves to be safe and effective, building the manufacturing capacity to make enough vaccine also takes time.
Unlike antibiotics, viruses and bacteria very infrequently become resistant to established vaccines. In the case of influenza (the flu), vaccine strains appear to change yearly through mutation or with new strains around the world and vaccine has to be adapted to the circulating strains every year.
However, most other vaccines do not experience major problems with resistance. The graphic below shows the time to detection of resistance to antimicrobials (in red) and to vaccines (in green). Source: Front. Immunol.
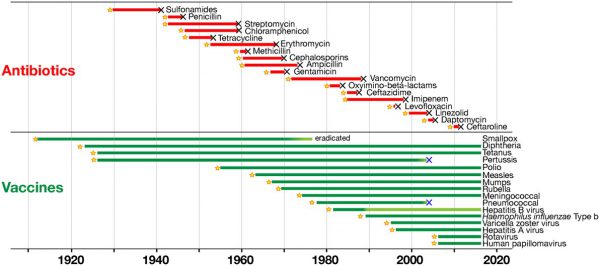
Vaccines are a sustainable solution to disease prevention and are sometimes used for large outbreaks of multidrug resistant bacteria, like Salmonella Typhi.
On the other hand, increasing resistance to antibiotics has become a major threat to global health, exacerbated by over- and misuse of antibiotics. Vaccination is an important tool against antimicrobial resistance by preventing infection in the first place and reducing (mis)use of antibiotics.
About IVI and COVID-19
Learn more about IVI’s COVID-19 program here.
Researchers, vaccine developers, funders, and manufacturers have come together to explore an array of vaccine candidates, streamline stages of clinical development, rapidly prepare clinical trial sites, expedite review processes, and ramp up global manufacturing capacity from the outset.
The graph below shows a more traditional 5-year vaccine development timeline at the top, and the accelerated 18-month timeline that may yield a safe and effective vaccine at unprecedented speed.
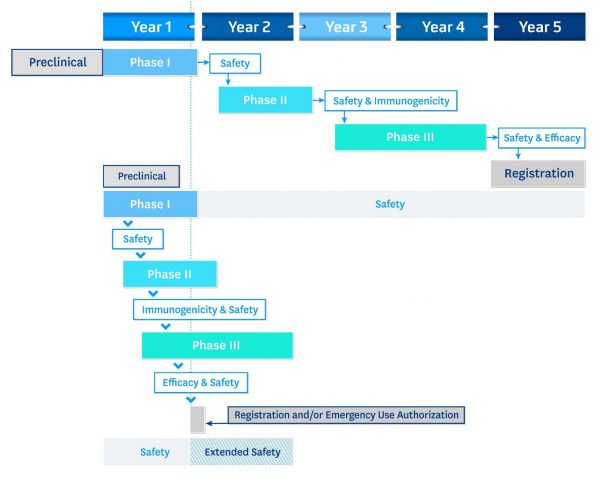
Accelerating vaccine development at the efficiency we have witnessed to date has been a globally collaborative effort of the kind required to end the pandemic.

