Chikungunya
Advancing the world’s first Chikungunya vaccine
About Chikungunya:
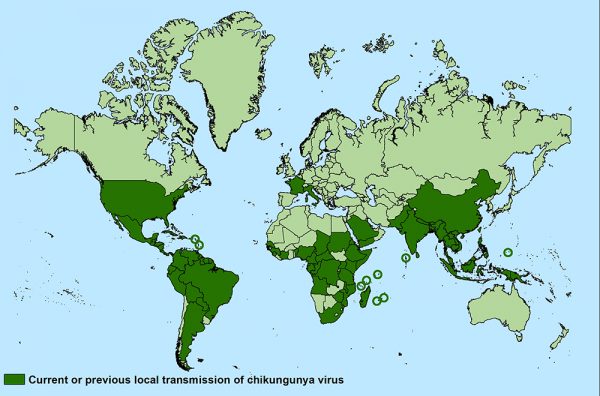
Chikungunya virus was first identified in Tanzania in 1952 with sporadic outbreaks of the disease reported across Africa and Asia. In 2004, the disease began to spread quickly, causing large-scale outbreaks around the world. Since the re-emergence of the virus, the total number of cases has been estimated at over 3.4 million in 43 countries.
Chikungunya is spread by the bites of infected female Aedes mosquitoes and causes fever, severe joint pain, muscle pain, headache, nausea, fatigue and rash. Although not a usually fatal disease, the resulting joint pain is often debilitating and can persist for weeks to years.
Why a vaccine?
Vaccination remains the most sustainable and cost-effective solution to protect one billion people from Chikungunya
There is no known cure or licensed vaccine to treat or protect against Chikungunya. Because the Chikungunya virus spreads through urban populations in mosquito-human cycles, transmission control relies on mosquito abatement, which is rarely effective.
Climate change could further amplify the threat posed by Chikungunya. As global temperatures rise, more areas around the world will become habitable for the mosquito vectors that transmit the virus, putting more people at risk of infection.
For example, in 2007, an outbreak of Chikungunya virus infections was declared for the first time in Europe with more than 200 human cases reported in Italy. In the United States, local transmission of the virus has been reported in Florida, Puerto Rico, Texas and the U.S. Virgin Islands since 2014.
What is IVI doing?
Accelerating a vaccine solution to prevent Chikungunya worldwide
IVI is leading the Global Chikungunya vaccine Clinical Development Program (GCCDP) consortium in partnership with Bharat Biotech and through the support of the Coalition of Epidemic Preparedness Innovations (CEPI) to conduct an adaptive Phase II/III vaccine clinical trial across three countries with the ultimate goal of achieving WHO pre-qualification.
In addition, the consortium will partner with the National Institute for Biological Standards and Control (NIBSC) in the UK to conduct non-human primate studies (on cynomolgous monkeys) to get additional insights on the immune response of this vaccine.

The study is a Phase II/III randomized, controlled trial with an adaptive and seamless design to evaluate the safety and immunogenicity of a 2-dose regimen of BBV87 Chikungunya vaccine in healthy subjects aged 12-65 years in Panama, Colombia, and Thailand. These studies are running in parallel to Bharat Biotech’s clinical trials in India.

GCCDP Grant Principal Investigator: Sushant Sahastrabuddhe
Study Principal Investigator: Anh Wartel
This study is supported by the Coalition for Epidemic Preparedness Innovations (CEPI).
Page last updated in January 2021