IVI and the People’s Republic of China
Member State Relations
On 13 January 1997, China signed the IVI Establishment Agreement.
On 18 August 1997, China deposited an instrument of approval to the IVI Establishment Agreement.
On 25 August 2020, the Embassy of China to the Republic of Korea donated $20,000 USD to IVI for vaccine research and development as well as efforts to advance international cooperation for vaccines against infectious diseases including COVID-19. H.E. Xing Haiming, the Ambassador of China to the Republic of Korea, visited IVI’s Headquarters to deliver the donation and meet with IVI Director General Jerome Kim.
Chinese Leadership at IVI
Prof. Chen Chunming (陈春明), Chinese Academy of Preventative Medicine, 1995-2000
Prof. Wang Ke An, Chinese Academy of Preventative Medicine, 2001-2003
Prof. Dr. Zhuang Hui (庄辉), Peking University Health Science Center, 2004-2007
Prof. Bin Wang (王斌), School of Basic Medical Sciences, Fudan University, 2018- Present
Ongoing Collaboration
Expanding COVID-19 Vaccine Access and Delivery in Africa (ECOVA) 1 & 2
Beginning in July 2021, ECOVA-01 is a Phase 3 randomized, observer-blind, controlled trial to assess the efficacy, immunogenicity, and safety of Sinopharm’s BBIBP-CorV vaccine against SARS-CoV-2 infection.
ECOVA-02 is a phase 2, observer-blind, randomized study to assess the safety, tolerability and immunogenicity of heterologous prime-boost COVID-19 vaccines regimens in healthy adults aged 18 to 65 years in Mozambique. ECOVA-02 is a mix and match study using the Sinopharm and Johnson & Johnson vaccines.
ECOVA 01 and 02 are conducted in cooperation with Mozambique’s Instituto Nacional de Saúde (INS) and made possible by a $12.7 million USD grant from the Coalition for Epidemic Preparedness Innovations (CEPI).
Hepatitis E Vaccine Roadmap
In collaboration with Johns Hopkins University and Syracuse University, IVI is contributing to the development of a roadmap to identify the key remaining barriers to wider available and use of the HEV vaccine Hecolin® produced by China’s Innovax Biotech. As part of this project, IVI will identify and prioritize clinical evidence gaps to inform policy and meet requirements (SAGE, WHO, and country-level) for routine and emergency use, while also assessing the readiness of Hecolin® for WHO prequalification and the status of other manufacturers with HEV vaccines in their development pipelines. Work began in mid-2021 with the first meeting of the Policy Advisory Group and Technical Advisory Group.
Previous Collaboration
IVI: Action across China
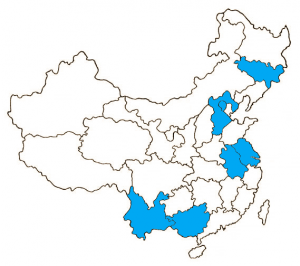
Anhui Province
• 2001-2003 Rotovirus/diarrhea study
Beijing
• 1999 Hib/meningitis study
• 2001-2003 Rotovirus/diarrhea study
Guangxi Province
• 2000-2007 DOMI program- Typhoid research and vaccination trial.
- 1998-2002 Hib/Meningitis incidence study
• Cohosted the 2015 International Symposium on Typhoid in Guilin
Hebei Province
• 2001-2003 Rotovirus/diarrhea study
• 2003 Shigella/dysentery disease burden and cost of illness studies
Jiangsu, Jilin, and Yunnan Provinces
• 2001-2003 Rotovirus/diarrhea studies
Shanghai
• 2002 Public health impact and cost-effectiveness studies on the Japanese Encephalitis vaccine
Zhejiang Province
• 1998 vaccine production workshop at Zhejiang Academy of Medical Sciences.
IVI: Award Winning Research in China
In 2001, IVI’s then lead scientist Dr. Xu Zhi Yi received China’s National Science and Technology Award(J-233-2-11) for his work on the Hepatitis A vaccine.

