Cholera
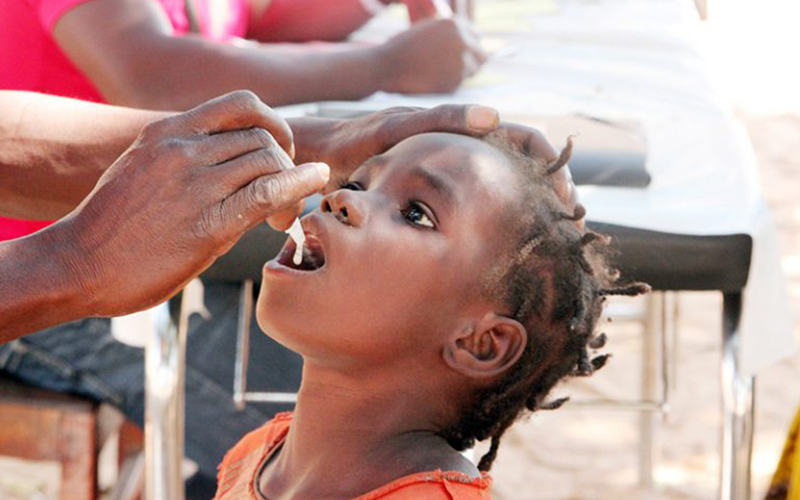 Cholera is an acute diarrheal infection caused by ingestion of food or water contaminated with the bacterium Vibrio cholerae. It affects both children and adults and can kill within hours if not properly treated. According to the WHO, there are 1.4 to 4.3 million cases and up to 143,000 deaths annually; with a disproportionate burden in Africa and South Asia.
Cholera is an acute diarrheal infection caused by ingestion of food or water contaminated with the bacterium Vibrio cholerae. It affects both children and adults and can kill within hours if not properly treated. According to the WHO, there are 1.4 to 4.3 million cases and up to 143,000 deaths annually; with a disproportionate burden in Africa and South Asia.
Cholera is a disease of poverty and strikes in settings where there is overcrowding and limited access to safe water and sanitation. Explosive and deadly outbreaks can occur following natural disasters and humanitarian crises when systems break down.
Improvements in water quality, hygiene and sanitation have been recognized as essential measures in preventing and controlling cholera. While effective, these are long-term measures and are challenging to implement in low-income countries with limited resources. Oral cholera vaccines can be an effective complementary tool that works in the short- to medium-term, especially during outbreaks when immediate action is needed.
Dukoral, a two-dose inactivated vaccine consisting of killed whole cells of Vibrio cholerae O1 and the B subunit of the cholera toxin, has been on the market since 1991. However, it is mainly used in developed countries as a traveler’s vaccine and has not been introduced in developing countries due to its relatively high cost.
 IVI’s Cholera Program aims to accelerate the development and introduction of safe, effective, and affordable oral cholera vaccines to combat epidemic and endemic cholera in developing countries.
IVI’s Cholera Program aims to accelerate the development and introduction of safe, effective, and affordable oral cholera vaccines to combat epidemic and endemic cholera in developing countries.
Through this program, we have developed a new low-cost two-dose killed whole-cell oral cholera vaccine. The vaccine (Shanchol) was prequalified by the WHO in 2011. Shanchol is currently stockpiled by the WHO for emergency use and has been used in nearly 20 countries such as Haiti, South Sudan, Malawi and Ethiopia. Read our story below to see how we accomplished bringing a vaccine to WHO prequalification – a major feat that few nonprofit organizations have achieved – and our ongoing efforts to combat cholera in the developing world.
Our cholera vaccine story
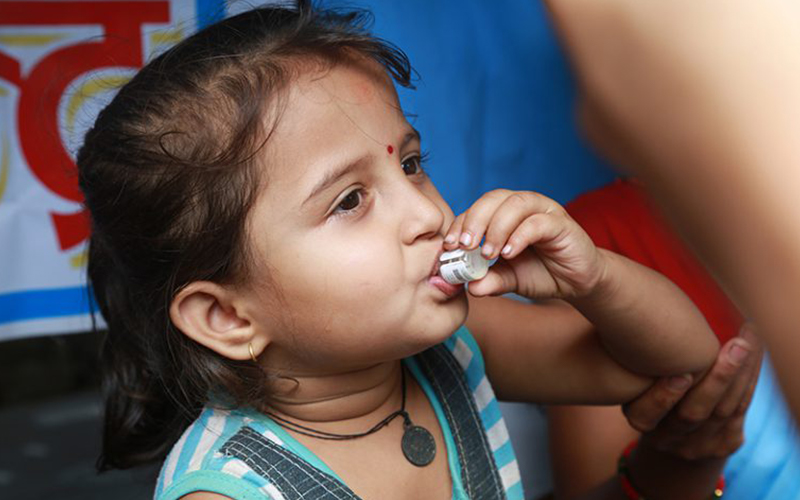 Development of the oral cholera vaccine was made possible due to an international public-private partnership led by IVI with partners from Sweden, Vietnam, India, Korea, and the U.S. In the early 2000s, Vietnam had already developed and licensed its own oral cholera vaccine. However, in order for the vaccine to be used globally, it had to be modified to meet WHO requirements. Therefore, in collaboration with VaBiotech, the Vietnamese manufacturer of the vaccine, IVI reformulated the vaccine and transferred the technology back to VaBiotech. The modified vaccine (mORC-VAX) was licensed in Vietnam and is now used nationally.
Development of the oral cholera vaccine was made possible due to an international public-private partnership led by IVI with partners from Sweden, Vietnam, India, Korea, and the U.S. In the early 2000s, Vietnam had already developed and licensed its own oral cholera vaccine. However, in order for the vaccine to be used globally, it had to be modified to meet WHO requirements. Therefore, in collaboration with VaBiotech, the Vietnamese manufacturer of the vaccine, IVI reformulated the vaccine and transferred the technology back to VaBiotech. The modified vaccine (mORC-VAX) was licensed in Vietnam and is now used nationally.
In addition, the technology was transferred to Shantha Biotechnics, a manufacturer in India – a country with a national regulatory authority (NRA) recognized by the WHO (a prerequisite for WHO prequalification). IVI worked with Shantha and other partners to perform clinical trials in Kolkata, India, to demonstrate that the vaccine was safe and effective. Since the vaccine also needed to be affordable to low-income countries, a global access agreement was made, in which Shantha agreed to provide the vaccine at an affordable price to the public sector. The pivotal trials conducted in Kolkata showed that two doses of the oral cholera vaccine had a protective efficacy of about 65 percent for at least five years – the first demonstration of strong, sustained protection by an oral cholera vaccine.
The vaccine was licensed in India as Shanchol in 2009, and IVI worked closely with Shantha on WHO prequalification, which was obtained in 2011. This was a major achievement for both global health and IVI. To date, Shanchol is one of only three WHO-prequalified vaccines that have come about through product development partnerships (PDPs).
Our Work Continues
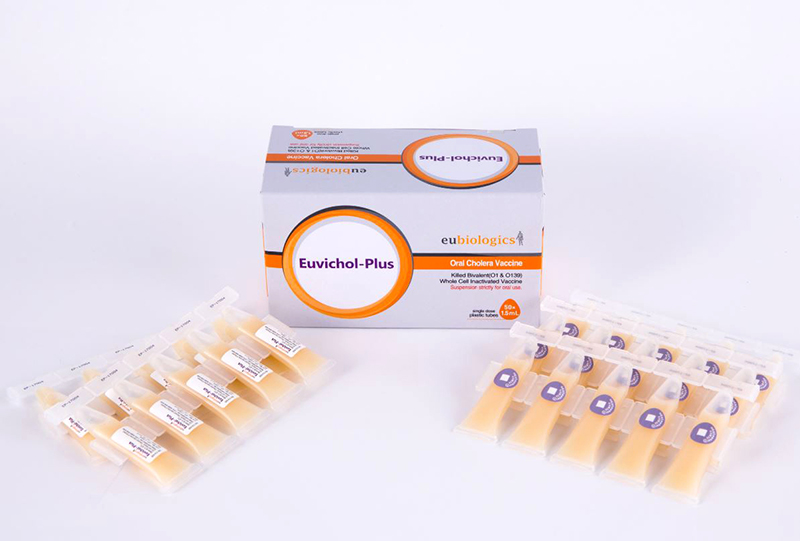 IVI recognized that more than one manufacturer was needed to ensure a reliable supply of the oral cholera vaccine for the global market. We therefore started working with EuBiologics, a Korean company, in 2010 as our second manufacturing partner. We have worked with Eubiologics to complete a technology transfer, implement the necessary trials for licensure in Korea, and obtain WHO prequalification. As part of the global access agreement, Eubiologics is also providing high-quality, affordable vaccines for public-sector use in resource-poor countries.
IVI recognized that more than one manufacturer was needed to ensure a reliable supply of the oral cholera vaccine for the global market. We therefore started working with EuBiologics, a Korean company, in 2010 as our second manufacturing partner. We have worked with Eubiologics to complete a technology transfer, implement the necessary trials for licensure in Korea, and obtain WHO prequalification. As part of the global access agreement, Eubiologics is also providing high-quality, affordable vaccines for public-sector use in resource-poor countries.
Substantial progress has been made on the EuBiologics vaccine, Euvichol®. The vaccine was WHO-prequalified in 2015. Once WHO prequalification was achieved, the vaccine could be sold through UNICEF to the global market, highlighting the major contribution of Eubiologics and Korea to the fight against cholera. Additionally, Euvichol-Plus®, which employs a user-friendly plastic container, was approved by the WHO Prequalification Program in December 2017. The addition of Euvichol-Plus® reduced the price of the vaccine and has increased global supply to more than 25 million doses for 2017, with the potential to increase production further in the future. More importantly, the extra capacity has contributed to reversing the cycle of low demand, low production, high price and inequitable distribution, to one of increased demand, increased production, reduced price and increased access. Euvichol-Plus®, priced at about $1.30 per dose, is 25 percent cheaper than Euvichol®, and it will therefore enable aid and vaccine delivery organizations to procure more doses of OCV at the same cost. This vaccine is being supplied to the WHO OCV stockpile.
More than 25 million doses of IVI’s OCV including Shanchol® and Euvichol (Euvichol-Plus®) doses have been administered in more than 25 countries worldwide.
In 2014, IVI also began working with another new partner, Incepta Vaccine Ltd, a Bangladeshi company, for the technology transfer and clinical development of the oral cholera vaccine. Since cholera is endemic in Bangladesh, there is great need for a domestically-produced oral cholera vaccine. The partnership with Incepta will help reduce the significant burden of cholera in Bangladesh.
IVI continues to work on optimizing the use of OCV in developing countries. For example, Shanchol consists of a two-dose regimen, but a single-dose regimen would be logistically easier to administer in campaigns for outbreaks and emergency situations. Therefore, a single-dose study was launched in Bangladesh at the beginning of 2014. IVI, with iccdr,b, recently published a study assessing whether the two-dose vaccine administered as a single dose provides sufficient protection against cholera.Learn more about the single-dose study published in the Lancet Infectious Diseases
IVI also continues to work in Vietnam, supporting efforts to pave the way for WHO prequalification of mORC-VAX. Because the Vietnamese NRA is now recognized by the WHO, mORC-VAX could be eligible for WHO prequalification in the near future.
Demonstrating impact
 A stockpile using the oral cholera vaccine was established in late 2013, supported by Gavi, the Vaccine Alliance and managed by the WHO and other partners. Initially, the purpose of the 2 million dose stockpile was to expedite the delivery of cholera vaccines for rapid outbreak response in emergencies and crisis situations. With the availability of additional doses, the stockpile now supports preventive campaigns in advance of an outbreak in communities at risk. From the initiation of the stockpile in 2013, through May 2018, around 25 million doses of OCV were deployed through campaigns in 19 different countries. While perceptions have been changing about using a vaccine to fight cholera, more evidence is needed to demonstrate the impact of vaccination and to generate demand for the oral cholera vaccine.
A stockpile using the oral cholera vaccine was established in late 2013, supported by Gavi, the Vaccine Alliance and managed by the WHO and other partners. Initially, the purpose of the 2 million dose stockpile was to expedite the delivery of cholera vaccines for rapid outbreak response in emergencies and crisis situations. With the availability of additional doses, the stockpile now supports preventive campaigns in advance of an outbreak in communities at risk. From the initiation of the stockpile in 2013, through May 2018, around 25 million doses of OCV were deployed through campaigns in 19 different countries. While perceptions have been changing about using a vaccine to fight cholera, more evidence is needed to demonstrate the impact of vaccination and to generate demand for the oral cholera vaccine.
IVI is collaborating with local health authorities and global health groups on introduction of the oral cholera vaccine to provide practical evidence that vaccination works, is feasible, and is well-accepted by the community. We provided technical support to icddr,b for the introduction of the oral cholera vaccine in Bangladesh, as part of a large-scale study that assessed vaccine effectiveness when given through the public health system. The results, published in The Lancet, showed that the vaccine was highly effective and added momentum for cholera vaccination.
With the support of local partners, we introduced the oral cholera vaccine in Nepal, Ethiopia, Malawi and Mozambique, vaccinating more than 400,000 people. The vaccine was introduced through campaigns in various settings (i.e.: a preventive campaign against endemic cholera in Ethiopia, and an emergency campaign against an outbreak in flood-stricken Malawi), showing that cholera vaccination is practical and feasible. Despite the progress made, our efforts are ongoing. IVI continues to work on cholera control, increasing vaccine supply, and advocating for the use of the vaccine – so that all at-risk communities are protected against cholera.
We gratefully acknowledge the Governments of the Republic of Korea, Sweden, India, the Bill & Melinda Gates Foundation, LG Electronics, Kia Motors, the Yanghyun Foundation, the Export Import Bank of Korea, Rotary International, and Kim & Chang for their support.

